Molecules - Signaling - Development
Research overview
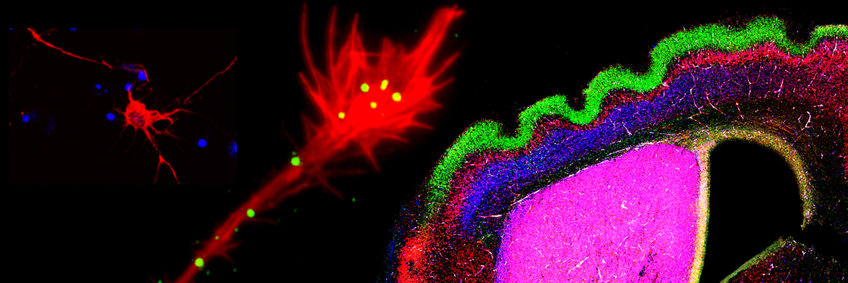
Our department investigates the common principles and functions of cell-cell communication in the developing and mature nervous system and the molecular mechanisms of neurodegeneration. Neuronal circuit formation is dependent on the precise navigation of nerve cells (neurons) and their cellular processes (axons and dendrites) to their final destinations. During development neurons explore their environment for the presence of biochemical signals that can be either attractive (adhesive) or repulsive. We are investigating the functions of these signals in a mammalian model organism, the laboratory mouse. Currently, our focus is on the development of the cerebral cortex (Project "Cell-cell communication in the developing brain).
Besides development, we are also interested in understanding how specific neuron populations communicate and contribute to certain types of behavior of adult mice. For these studies, we use genetic methods to gain access to specific microcircuits, we record the activities of well-defined neuron populations in freely behaving mice, we use opto- and pharmacogenetic tools to reveal the functions of these neurons, and we anatomically map the microcircuits. By doing so, we gain insight into how the brain works at the level of individual cell populations. Currently our focus is on amygdala and spinal cord (Project "Circuit dynamics and behavior").
In the context of an ERC-funded project, we are studying the molecular mechanisms of neurodegeneration. Many age-dependent neurodegenerative diseases are characterized by deposition of damaged proteins in the brain. We are using mouse models of human diseases, currently with a focus on Huntington’s disease, to study the effects of these deposits on different types of nerve cells, as well as the mechanisms used by the cells to reduce the accumulation of damaged proteins (Project "Molecular mechanisms of neurodegeneration").


